Protein Photonics Special Section In Journal Of Biomedical Optics Honors Nobel Laureate Osamu Shimomura
Discovery of green fluorescent protein paved the way for light-activated study and treatment of the brain
A special section on Protein Photonics in the Journal of Biomedical Optics celebrates the accomplishments and influence of Nobel Laureate Osamu Shimomura. His work in isolating green fluorescent protein (GFP) from jellyfish paved the way for numerous applications of fluorescent proteins in imaging of living tissue and in biological microscopy.
Guest editors of the special section are Katsumasa Fujita and Takeharu Nagai of Osaka University,Nathan Shaner of The Scintillon Institute, and Alexander Egner of Georg-August-Universität Göttingen.
Professor Shimomura’s work provided inspiration for the idea of using light-protein interactions in fields such as optogenetics, where cell and animal activities are manipulated and imaged through the application of light on chemically treated brain cells, note the special section guest editors in their editorial published today.
Fluorescent proteins are used to tag proteins and structures in live cells and tissues, and to report on intracellular characteristics such as pH and temperature. In combination with other techniques, fluorescent proteins enable researchers to follow molecular-level activity in live cells.
In 1961, Shimomura and Frank Johnson, his mentor at Princeton University, extracted and purified luminescent protein material from the jellyfish Aequorea Victoria. They discovered that the protein, now known as GFP, absorbs blue light while emitting green when radiated with ultraviolet (UV) or blue light.
First cloned in 1994, GFP has enabled researchers to observe processes that were previously invisible, such as the development of nerve cells in the brain or the spread of cancer cells. By using GFP to tag specific proteins, scientists have studied how nerve cells are damaged by Alzheimer's disease and how insulin-producing beta cells are created in the pancreas of a growing embryo.
Now Professor Emeritus at the Marine Biological Laboratory in Woods Hole and Boston University Medical School, Shimomura shared the Nobel Prize in Chemistry 2008 with Martin Chalfie, who successfully introduced the gene for GFP into the DNA of other live organisms, allowing GFP to be used as a luminous genetic tag, and Roger Tsien, who determined how GFP's structure produces green fluorescence, then tweaked the structure to produce molecules that emit light at slightly different wavelengths, creating tags of different colors.
“Development of red-shifted mutants derived from luciferase of Brazilian click beetle Pyrearinus termitilluminans” describes work with a bioluminescent protein that emits red light, useful because of its high transmittance in mammalian tissues. Tomoki Nishiguchi and co-authors at the University of Tokyo report that four amino acid mutations introduced into Emerald luciferase from the Brazilian click beetle caused an 88-nm red-shift from the wild-type luciferase, making the two types (the mutant and the wild type) suitable for dual-color imaging on living samples.
In the open-access article “Correcting spherical aberrations in a biospecimen using a transmissive liquid crystal device in two-photon excitation laser scanning microscopy” Ayano Tanabe of Hokkaido University and co-authors describe a method to improve spatial resolution and the yellow fluorescent protein signal of the visualization of deep regions in a biospecimen.
Masahito Yamanaka and other researchers at Osaka University present a new method of multicolor imaging through the concurrent excitation of multiple fluorescent proteins, using green laser light rather than deep UV light, in “Visible-wavelength two-photon excitation microscopy for fluorescent protein imaging” -- also available via open access.
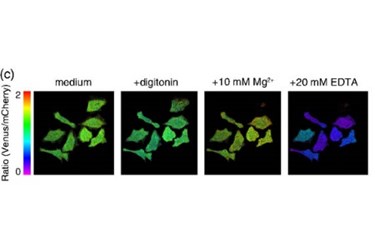
In “MagIC, a genetically encoded fluorescent indicator for monitoring cellular Mg2+ using a non-Förster resonance energy transfer ratiometric imaging approach,” Vadim Pérez Koldenkova and other Osaka University researchers explain how an indicator protein -- composed of a functionalized yellow fluorescent protein variant fused to a red-emitting fluorescent protein -- can be used to monitor magnesium ions in specific organelles at the subcellular level.
Norio Yamashita of RIKEN, 2014 Nobel Laureate in Chemistry Eric Betzig of the Howard Hughes Medical Institute, and their co-authors contributed "Three-dimensional tracking of plus-tips by lattice light-sheet microscopy permits the quantification of microtubule growth trajectories within the mitotic apparatus."
Lihong Wang, Gene K. Beare Distinguished Professor of Biomedical Engineering at Washington University in St. Louis, is editor-in-chief of the Journal of Biomedical Optics. The journal is published in print and digitally in the SPIE Digital Library, which contains more than 430,000 articles from SPIE journals, proceedings, and books, with approximately 18,000 new research papers added each year.
About SPIE
SPIE is the international society for optics and photonics, an educational not-for-profit organization founded in 1955 to advance light-based science and technology. The Society serves nearly 264,000 constituents from approximately 166 countries, offering conferences and their published proceedings, continuing education, books, journals, and the SPIE Digital Library in support of interdisciplinary information exchange, professional networking, and patent precedent. SPIE provided more than $4 million in support of education and outreach programs in 2014. SPIE is a Founding Partner of the International Year of Light and Light-based Technologies and a Founding Sponsor of the U.S. National Photonics Initiative.
Source: SPIE
